| ** | Latin American Journal of Clinical Sciences and Medical Technology is an open access magazine. To read all published articles and materials you just need to register Registration is free of charge. Register now If you already have registered please Log In | ** |
aHaut Kinik, Mexico City, Mexico; bMLG Media C.A. “A tu Salud”, Caracas, Venezuela; cERSA Medical Aesthetics, Monterrey, Nuevo León, México; dHospital San Juan de Dios, San José, Costa Rica; eProteos Biotech, Madrid, España.
Corresponding Author: , . Telephone number: ; e-mail: draingridgehrkehk@haut.mx
Lat Am J Clin Sci Med Technol. 2024 Mar; 6: 47 - 55.
Received: March 26th, 2024.
Accepted: April 3rd, 2024.
Published: April 8th, 2024.
Views: 267
Downloads: 2
Introduction. The recombinant enzymes, produced through biotechnological processes, have different medical applications. Recently, though poorly documented, they have been applied in aesthetic medicine to improve certain conditions that significantly affect patients' appearance, image, and quality of life. Objective. To document the use of recombinant enzymes in cosmetic dermatology regarding indicators of patient satisfaction with the procedure. Material and Methods. In this article, a series of cases have been brought together to document the use of recombinant enzymes in managing flaccidity and fat accumulation in certain facial and body areas, including measuring satisfaction with the results. Results. Two hundred twenty-nine cases from a Latin American multicenter trial were documented from private clinical practice using a standardized data collection format and a guide for clinical photography. Discussion. Our findings in 229 Latin American cases show a high grade of satisfaction in the management of double chin, jowl, abdominal flaccidity, and flaccidity in the limbs, as well as a good aesthetic outcome with the use of the combination of collagenase, hyaluronic-lyase, and lipase. Conclusions. The use of recombinant enzymes in managing the indications studied in clinical practice in cosmetic dermatology and aesthetic medicine, based on their effects on tissues, both on the face and the body, is highly satisfactory, and their results are well evaluated.
Introducción. Las enzimas recombinantes, producidas mediante procesos biotecnológicos, tienen diversas aplicaciones en medicina. Una de ellas, reciente y poco documentada, se usa en dermatología cosmética para mejorar ciertas condiciones que afectan significativamente el aspecto, imagen y calidad de vida de los pacientes. Objetivo. Documentar el uso de enzimas recombinantes en dermatología cosmética, en cuanto a los indicadores de satisfacción del paciente con el procedimiento. Material y métodos. En este artículo se ha reunido una serie de casos que documentan el uso de enzimas recombinantes en el manejo de la flacidez y acúmulo de grasa en ciertas zonas faciales y corporales, incluida una medición de la satisfacción con los resultados. Resultados. Se documentaron 229 casos en un estudio multicéntrico latinoamericano, provenientes de la práctica clínica privada mediante un formato de recolección de datos estandarizado y una guía para la fotografía clínica. Discusión. Nuestros hallazgos en 229 casos latinoamericanos muestran un alto grado de satisfacción en el manejo de doble mentón, jowl, flacidez abdominal y flacidez de extremidades, así como un buen resultado estético con el uso de la combinación de colagenasa, hialuronidasa y lipasa recombinantes. Conclusiones. Con base en sus efectos en los tejidos, el uso de enzimas recombinantes en el manejo de las indicaciones estudiadas de la práctica clínica en dermatología cosmética y medicina estética (tanto en cara como en el cuerpo) es altamente satisfactorio y sus resultados son bien evaluados.
Enzymes are proteins found in live organisms and catalyze, that is, accelerate, the speed of many biochemical reactions necessary to sustain life.1 With recombinant DNA technology, it is possible to clone genes that codify these enzymes and express them heterologically on bacterial strains commonly used in drug production.2
Enzymatic therapy is a new method used in aesthetic dermatology and medicine. It is the injection of enzymes such as lipase, collagenase, hyaluronidase, and liase into the skin and subcutaneous tissue to reduce local fat and remodel collagen.3 When these recombinant enzymes of non-animal origin are applied, the risk of allergic reactions is minimized because they are stored without adding preservatives; they are non-glycosylated molecules.
Every enzyme acts only on its substrate without affecting other substances or structures.4 Lipase degrades those triglycerides in adipose tissue cells, reducing their volume. Collagenase breaks nonfunctional and senescent collage, enhancing the neosynthesis of functional collagen fibers, which explains a flabby skin tightening effect.3 Hyaluronate liase and other liases degrade polysaccharides, glycosaminoglycans, and proteoglycans, increasing density and liquid retention on the extracellular matrix in certain areas.
Pbserum recombinant enzymatic systems have different combinations of these three enzymes, depending on the indication for treatment. For example, pbserum medium is applied in several small injections to treat submental fat. This procedure must be done two to five times with an interval of approximately 2-3 weeks. After each session, a gradual fat reduction is observed in area.5
Enzymatic therapy has been extensively used in dermatology and aesthetic medicine, especially in managing submental fat (double chin) and jowl (skin bags from the cheeks that, due to flabbiness, hang over the mandible line or profile). It has also been used in the management of abdominal flabbiness, which is common in women after giving birth or in patients who have lost weight in a significant way, and for the treatment of localized body fat.
Professionals in aesthetic dermatology and medicine observe that such clinical conditions significantly affect these patients' image, confidence, and quality of life. Therefore, new therapeutic approaches must be researched, documented, have their use standardized, and disclose the results.6
The procedure is minimally painful, and it consists of applying an anesthetic cream on the relevant area before the procedure and then injecting the enzymatic solution several times with a fine needle. Erythema and edema caused by the infiltration disappear a few hours after the procedure (rarely lasts more than 1-2 days). A bruise can form on the application site but disappears after a few days.
Fat reduction is achieved while the effect of each procedure adds and opts for a healthy lifestyle.
Massaging the area is recommended for a couple of days after the procedure to ease the lymphatic drainage, drinking plenty of water, limiting carb and fat intake, reducing physical activity for 48 hours, and avoiding sauna, swimming, and UV7 sunlight exposure.7
Due to a lack of publications documenting its effectiveness, safety, and degree of satisfaction, we decided to put together a series of cases from the clinical aesthetic dermatology and medicine practice. Doctors, users of enzymatic therapy, were asked to include a case from their practice and to fulfill a data harvesting form to follow the patient's evolution and satisfaction. Our research question was: what is the degree of satisfaction and the effectiveness and safety of pbserum medium in managing flabbiness and fat accumulation in face and body areas?
Two hundred twenty-nine clinical cases managed by the same number of doctors that use recombinant enzymes in their aesthetic dermatology and medicine practice were gathered (between 2019 and 2020) at private clinics from Mexico City, Monterrey (Mexico), Caracas (Venezuela), and San José (Costa Rica). They used the same clinical history and data harvesting form to apply enzymes. Patients were asked to fill out an informed consent.
With the results found in daily clinical practice, empirically, the patient’s degree of satisfaction was expected to be high. The working hypothesis was that the degree of satisfaction, effectiveness, and safety of pbserum medium in managing flabbiness and fat accumulation in face and body areas could be demonstrated. The final assessment was made 30 days after the last session.
The form harvested data such as age, gender, pathological background, indication for the elected procedure, description of the procedure, used enzymes, number and dates of sessions, and baseline and final photos, according to a standardized photo guide. It also included the treating doctor’s conclusions, their general impression of the result (classified as favorable change, without change, or unfavorable change), and the patient’s satisfaction with the result (satisfied or unsatisfied).
As a motive for consultation, patients must manage double chin, jowl or mandible fat, flabbiness, and located fat. Every patient signed a rights cession and acceptance letter for the use of their pictures for academic purposes. Data was captured ad hoc and underwent a descriptive statistical analysis.
The preparation technique for the pbserum medium product for double chin and jowl consists of obtaining, in reconstitution, a final volume of 6ml.
Application on the double chin
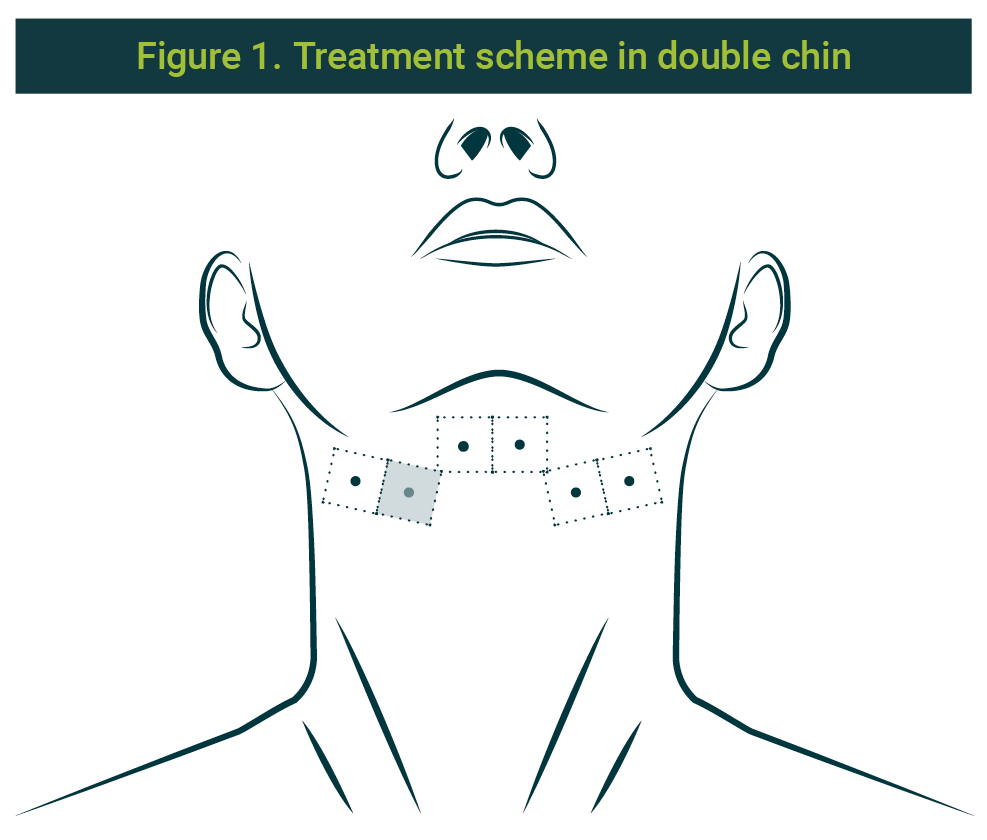
Previous informed consent obtained from the patient, and the diagnosis of volume and location of the preplastimal fat; asepsis and antisepsis of the area under the chin were made. 1 ml was applied, distributed on each point: 0.5ml on the profound level (subcutaneous) and 0.5ml on the superficial level (intradermic) of the combination of collagenase, hyaluronate lyase, and lipase, already prepared, on 6 points, separated 1 cm each; 2 left laterals, 2 centrals (parallels, behind the chin and before the hyoid) and 2 right laterals). The used needle size was 30G × ½ (13mm). Three sessions were performed, with a two-week or 15-day interval between them. The scheme can be seen in Figure 1.
Application on jowl
Previous informed consent from the patient and asepsis and antisepsis of the area, the recombinant enzymes pbserum (lipase, collagenase, hyaluronate lyase) in 3 applications of 1 ml on each side in the profound level (subcutaneous) of the combination of collagenase, hyaluronate lyase, and lipase, already prepared, into the jowl.
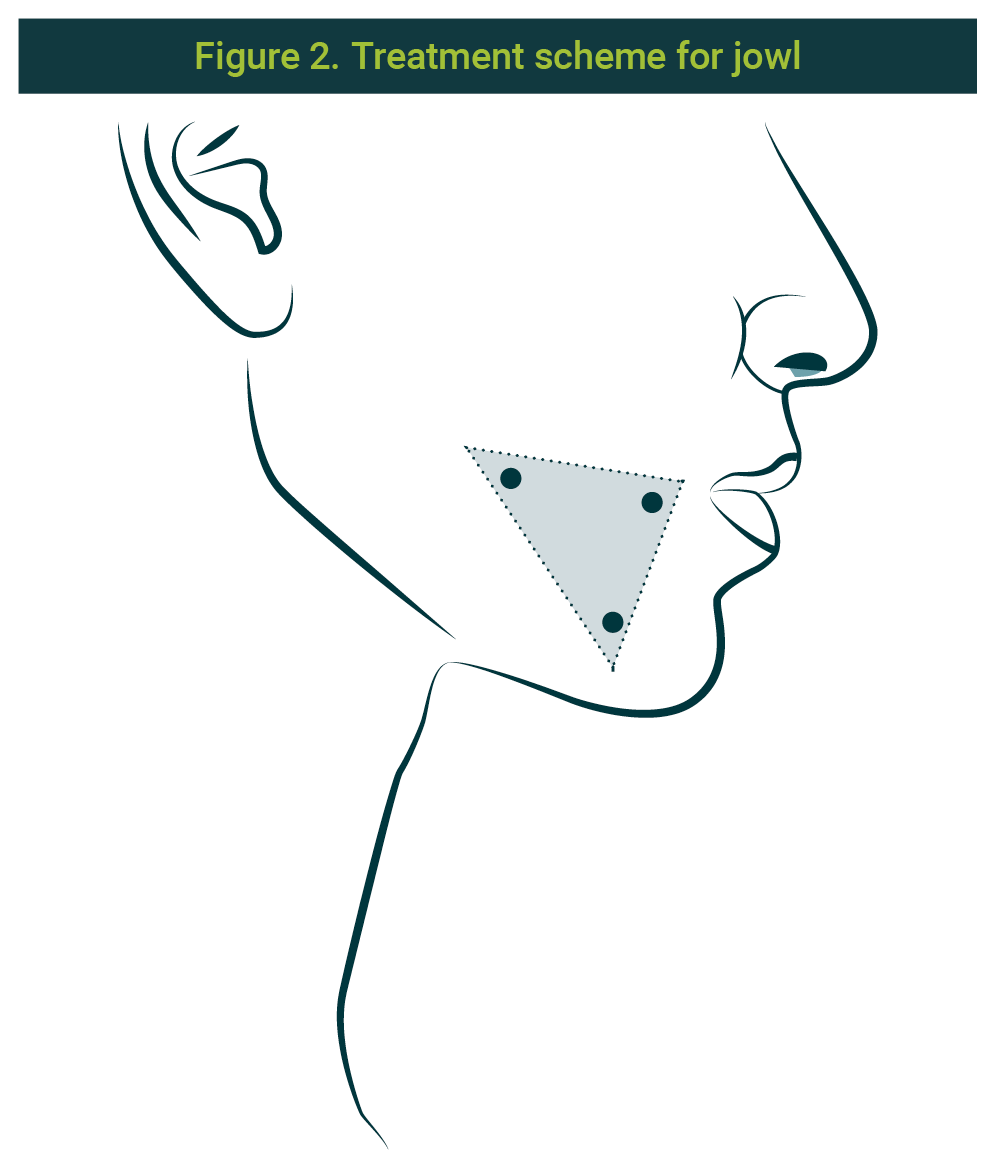
Points were marked with a distance of 1 cm between them. Three anatomical references were used; a triangle was designed taking as the anterior vortex a point 1 cm behind the lip commissure, another point (inferior vortex) placed on the inferior edge of the mandibular arch, and the superior vortex 1 cm higher. The scheme can be seen in Figure 2
Application on abdominal fat
The preparation technique for pbserum medium product for its abdominal application consists of obtaining, with reconstitution, a final volume of 20 ml.
Application on localized body fat
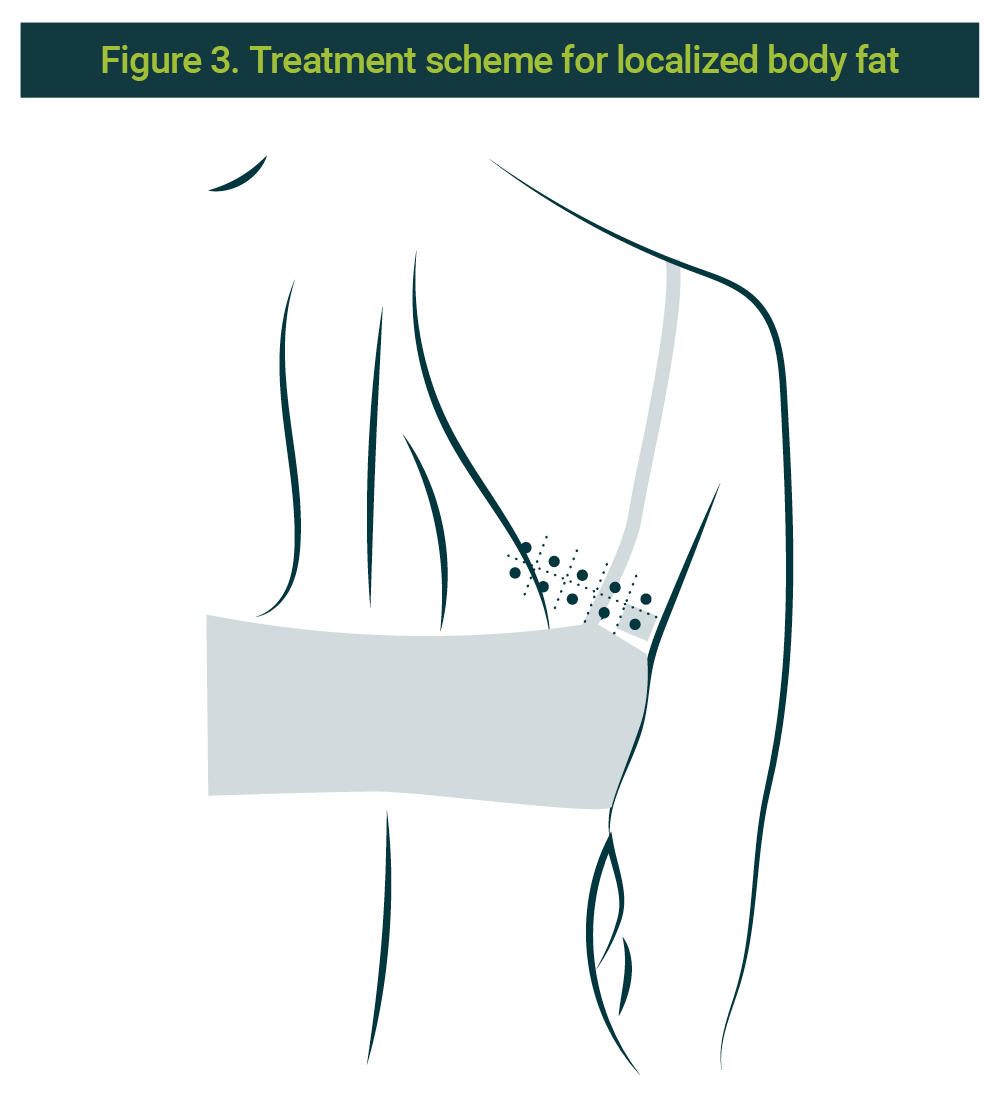
Previous informed consent from the patient as well as asepsis and antisepsis of the area, the application of pbserum of recombinant enzymes is done (lipase, collagenase, hyaluronate lyase) on 10 points at a profound level (subcutaneous), distributed in three sessions with a 15 days interval between them. The 10 points correspond to a contralateral zone of the treated area. Each side is marked with a grid of 120 cells by side, no bigger than 10 x 10 cm. A 30G × ½ (13mm) needle was used. The scheme is illustrated in Figure 3.
Clinical cases were divided into the different indications for recombinant enzymes in aesthetic medicine: double chin, jowl, abdominal flabbiness, limb flabbiness, and cases of combined indications such as jowl and double chin in the same session. The number of cases by indication is shown in Table 1.
| Table 1. Clinical cases | |
|---|---|
| Indication | Total |
| Double chin | 172 |
| Jowl | 23 |
| Localized fat and abdominal flabbiness | 19 |
| Limb flabiness | 7 |
| Combined | 8 |
| Total | 229 |
Double chin
One hundred and seventy two cases of this indication were gathered. The mean age was 36.5 years, with a maximum age of 66 and a minimum of 20. Forty (23.25%) were men, and 132 (76.75%) were women.
The surgical background in this group was irrelevant and unrelated to aesthetic dermatology and medicine procedures, except for one case of cheek lift and one of liposculpture, both without complications.
Regarding a history of chronic degenerative conditions, we found 3 cases of type 2 diabetes mellitus, 2 cases of dyslipidemia, and 7 patients with hypertension. Five patients had different allergies, and 3 had a history of recent COVID-19 infection.
All double chin cases were managed with a combination of collagenase, hyaluronate lyase, and lipase. In 88.3% of the cases, a 9 ml dose of the combination was used. In 87.7%, two sessions were performed. Eight cases required three sessions. The sessions needed for double chin treatment are shown in Table 2.
| Table 2. Sessions | |
|---|---|
| Session | Cases |
| 1 | 4 |
| 2 | 151 |
| 3 | 8 |
| 4 | 7 |
| 5 | 1 |
| 6 | 1 |
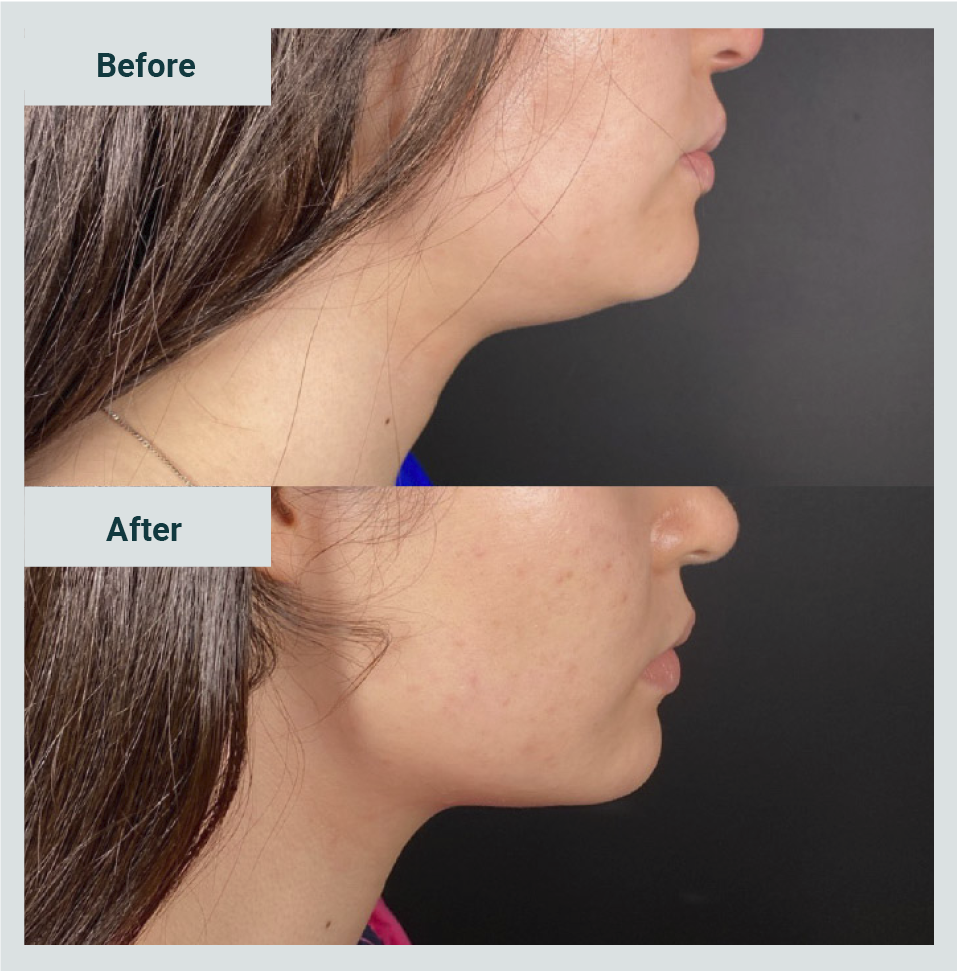
The most frequent interval between sessions was weekly. In Picture 1, one can see the example of a participating patient and the results observed with the application of two weekly sessions.
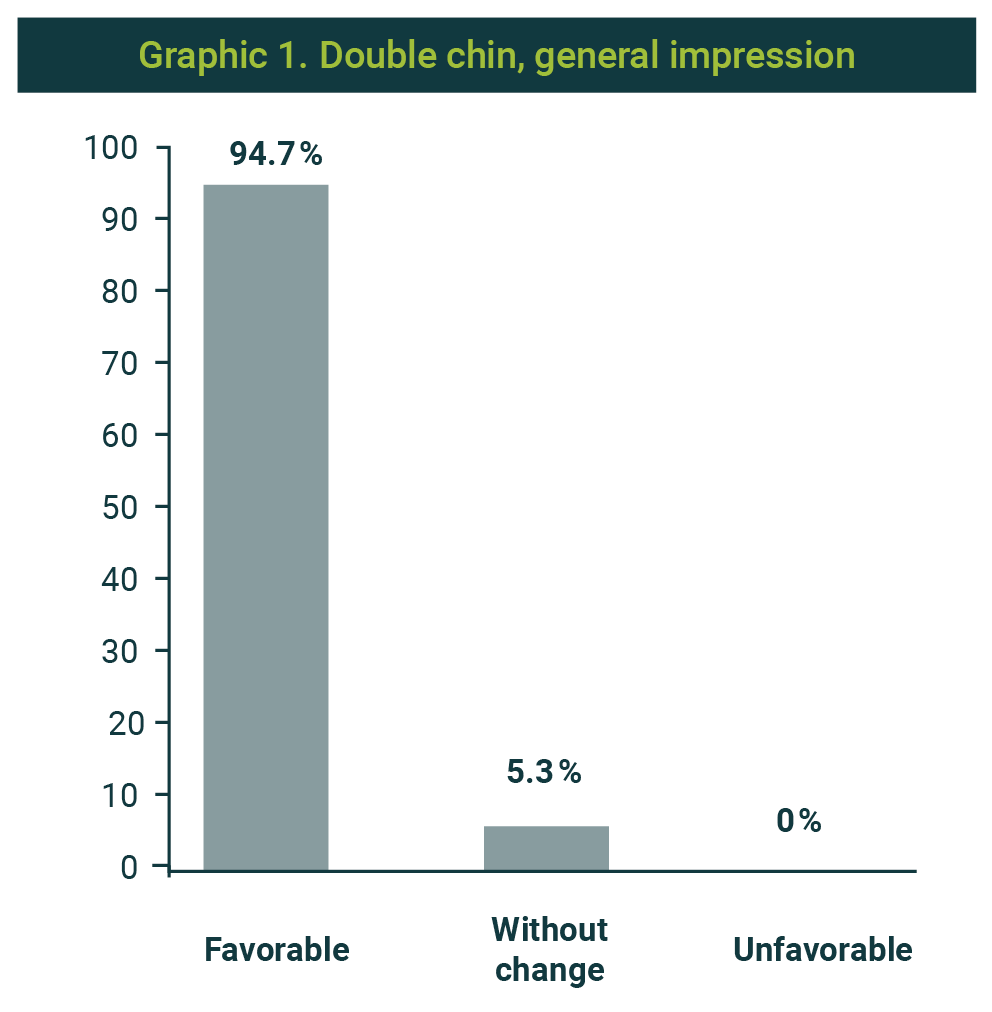
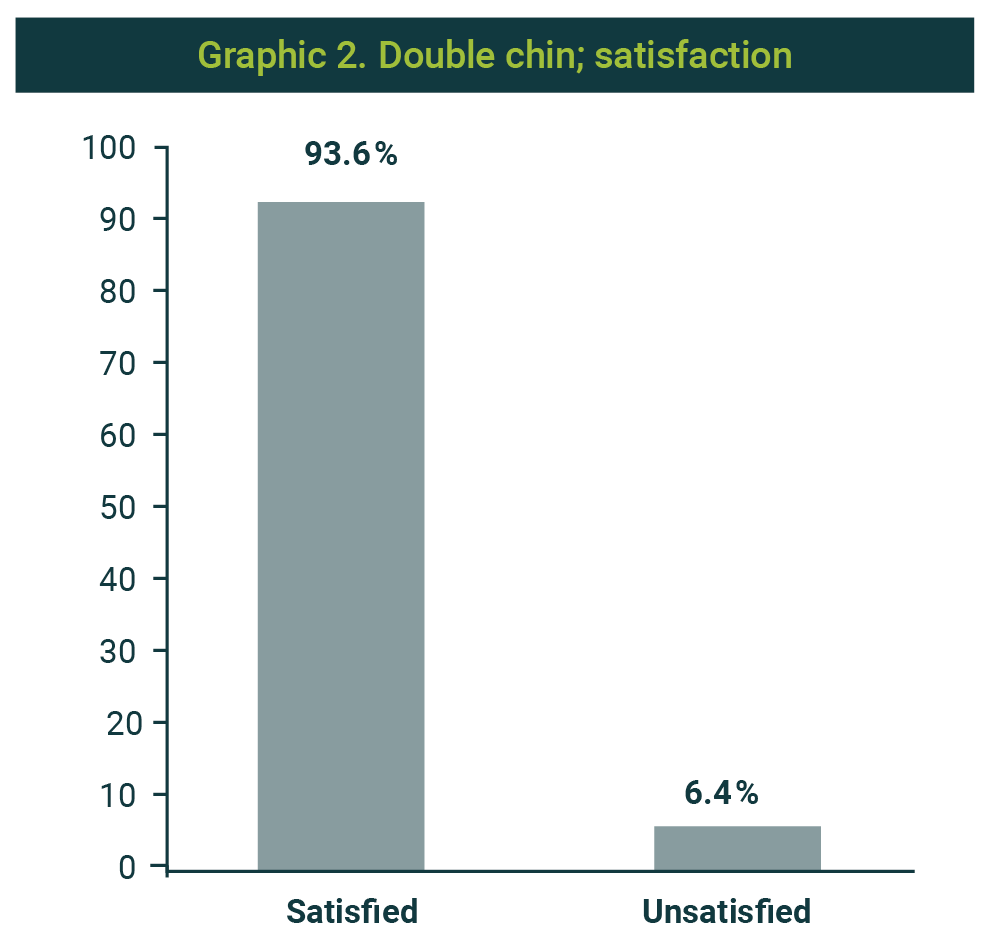
Regarding the treatment's general impression, in 163 cases (94.7%), the treating doctor assessed it as favorable change; 9 cases were reported as without change and none as unfavorable (Graphic 1). Patients' satisfaction with the procedure was positive (satisfied) in 93.6% (Graphic 2). Only 11 cases were assessed as unsatisfactory.
Jowl
In managing jowl, 23 cases were obtained, 10% of all the gathered cases. This segment's mean age was 43; 21 cases were women, and 2 were men.
One case had a history of cheek lift; one case had hypertension; another one had type 2 diabetes mellitus and dyslipidemia. No allergic history was found.
In all cases, treatment combined collagenase, hyaluronate lyase, and lipase. The predominant dose was 6 ml; in 82.6%, two sessions were applied. The preferred interval between sessions for jowl treatment was weekly (86.9%).
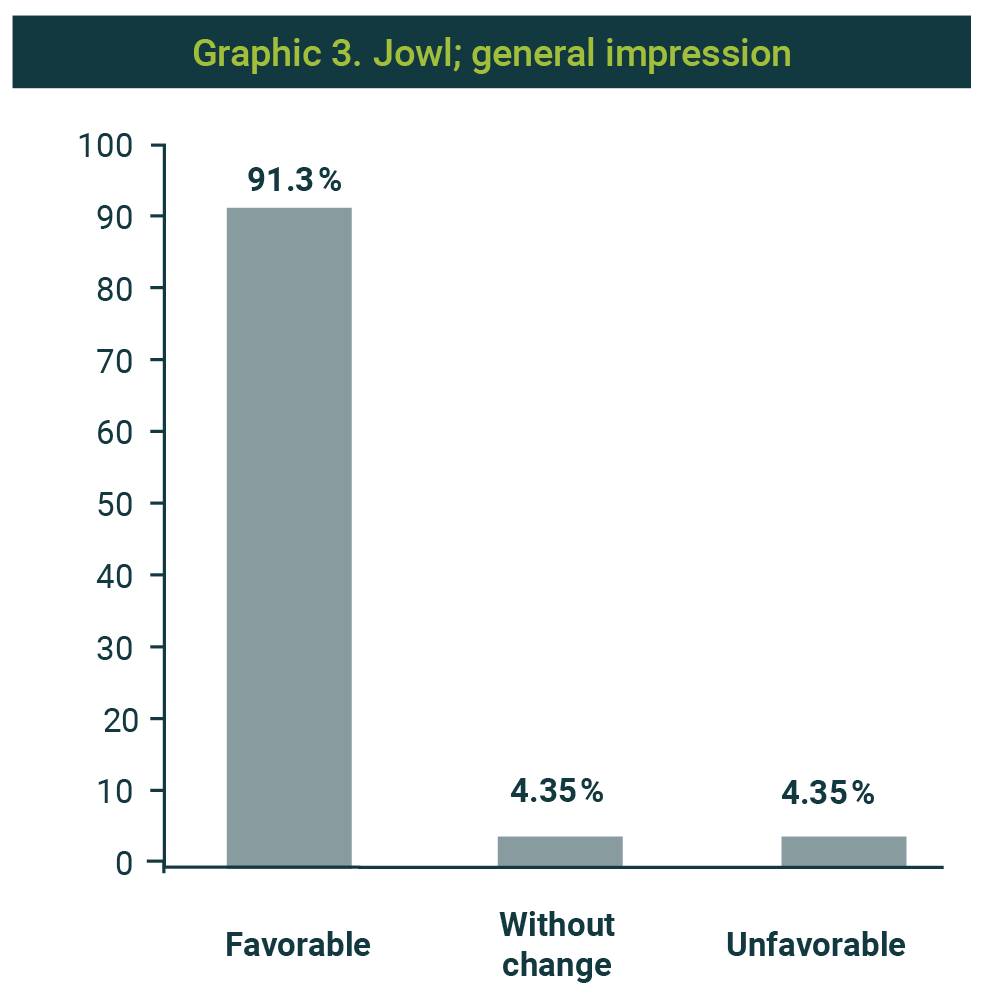
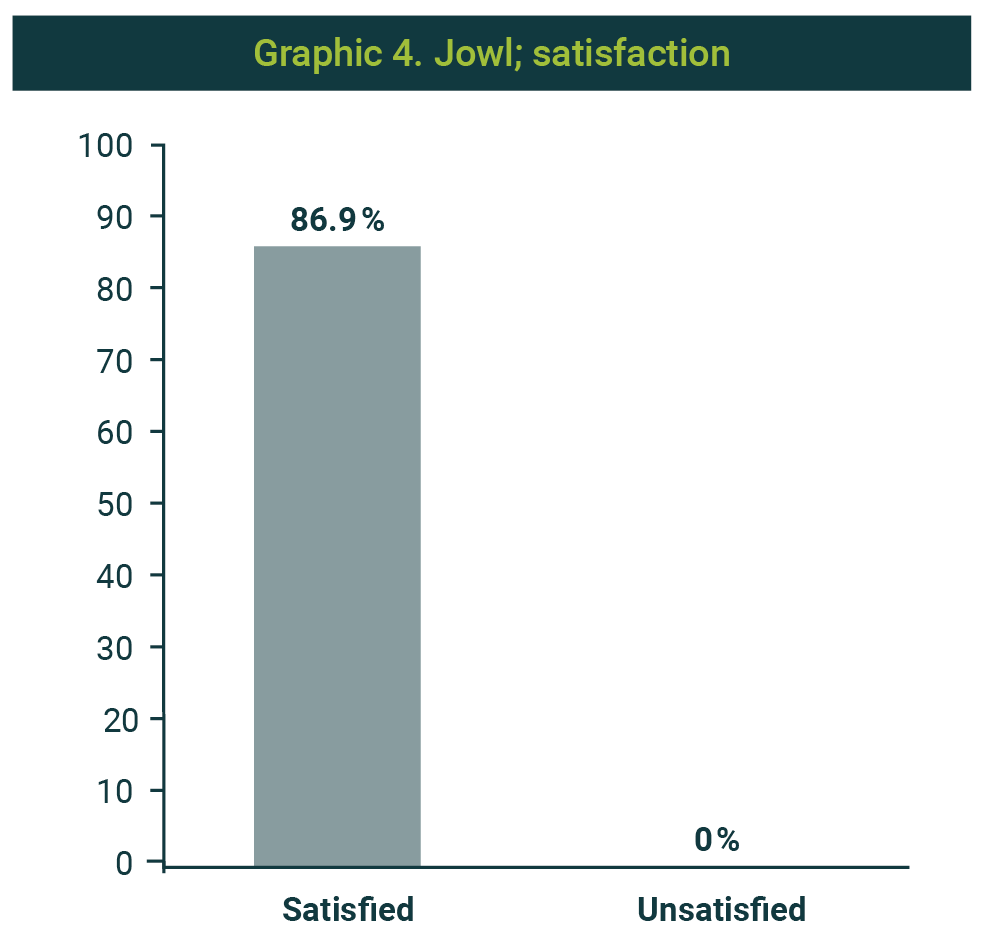
The favorable impression of the procedure's result was 91.3%; one case was reported without changes and another with unfavorable changes (Graphic 3). In 20 cases (86.9%), satisfaction with enzymatic management was reported (Graphic 4). Picture 2 shows the observed results with the application of two weekly sessions.
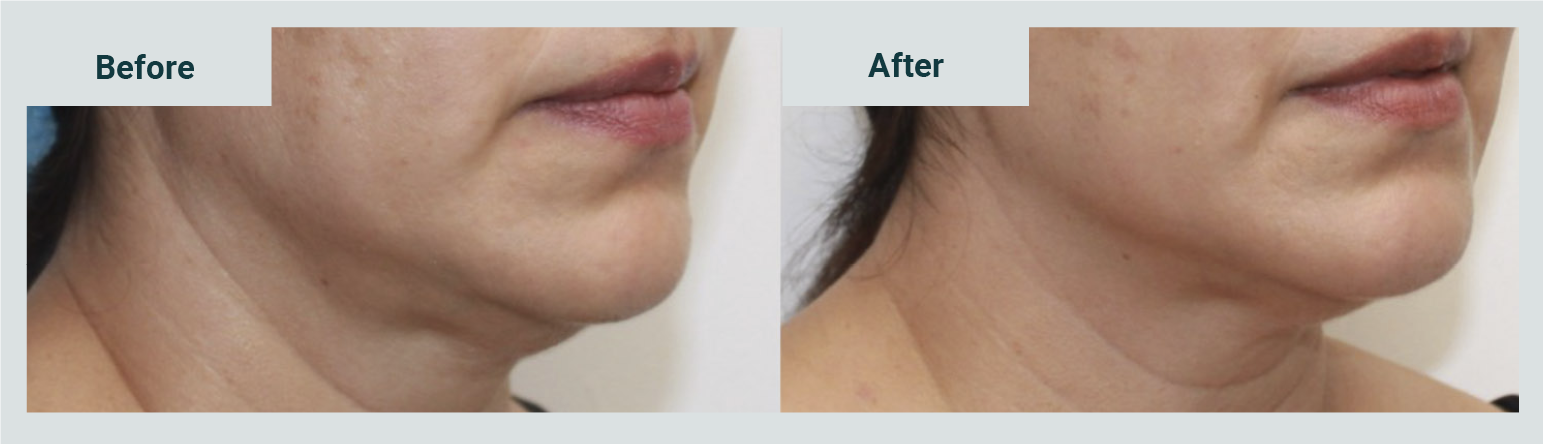
Abdominal flabbiness and localized fat
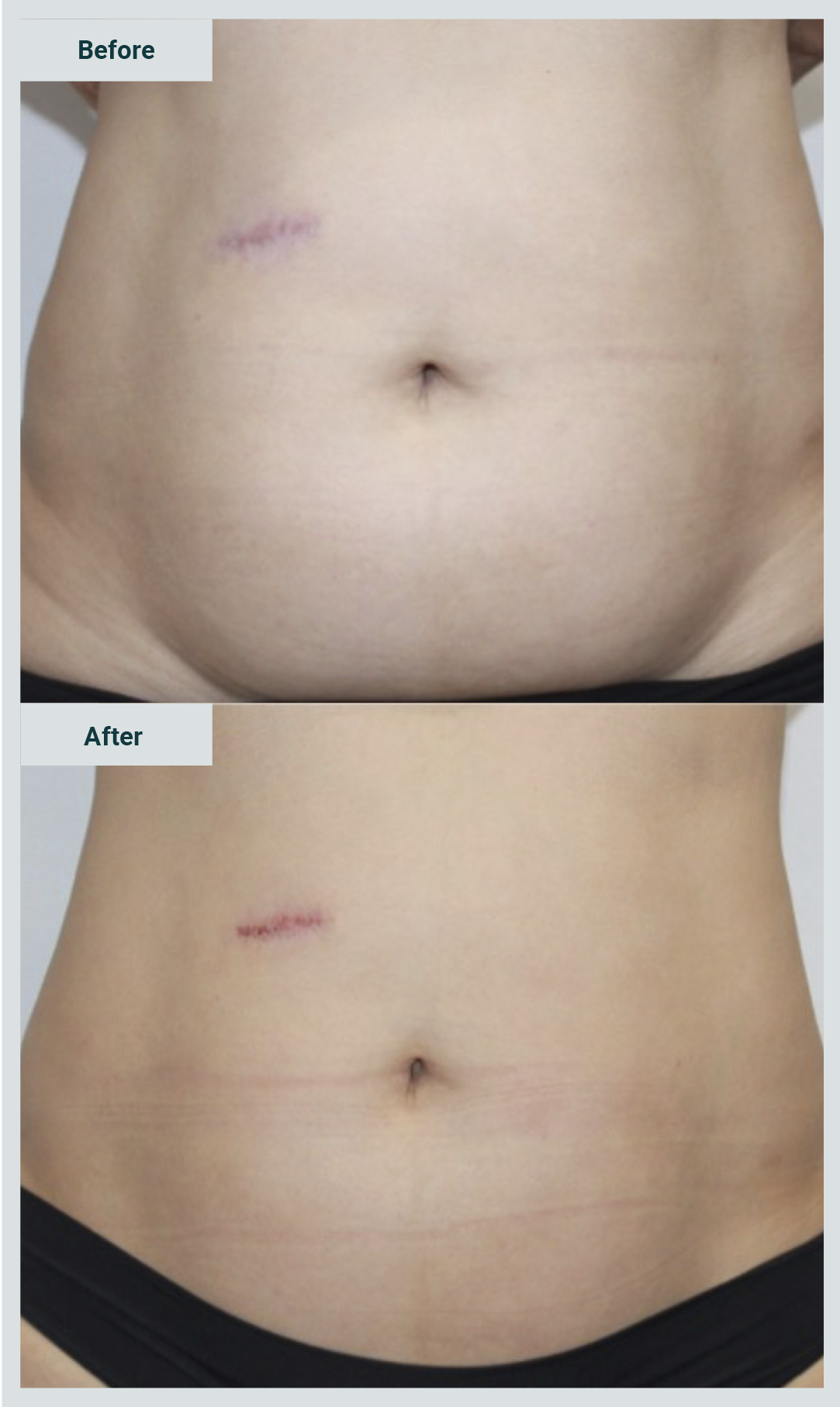
Nineteen cases were obtained (8.3% of all the cases in the series); the mean age of the patients was 40.5 years, and 17 cases were women and 2 were men.
One case had a surgical history of abdominoplasty and 2 of liposuction. Regarding pathological background, one had hypertension, and another one had hypothyroidism. No allergic background was found in any patient.
The combination of pbserum recombinant enzymes used was mostly collagenase, hyaluronate lyase, and lipase in 18 cases, and collagenase and lipase in one case. The most common volume used (73%) was 12ml; in 15 cases (78.9%), four sessions were required. In one case, 11 sessions were performed. The interval between sessions was weekly for 73.6% of the cases.
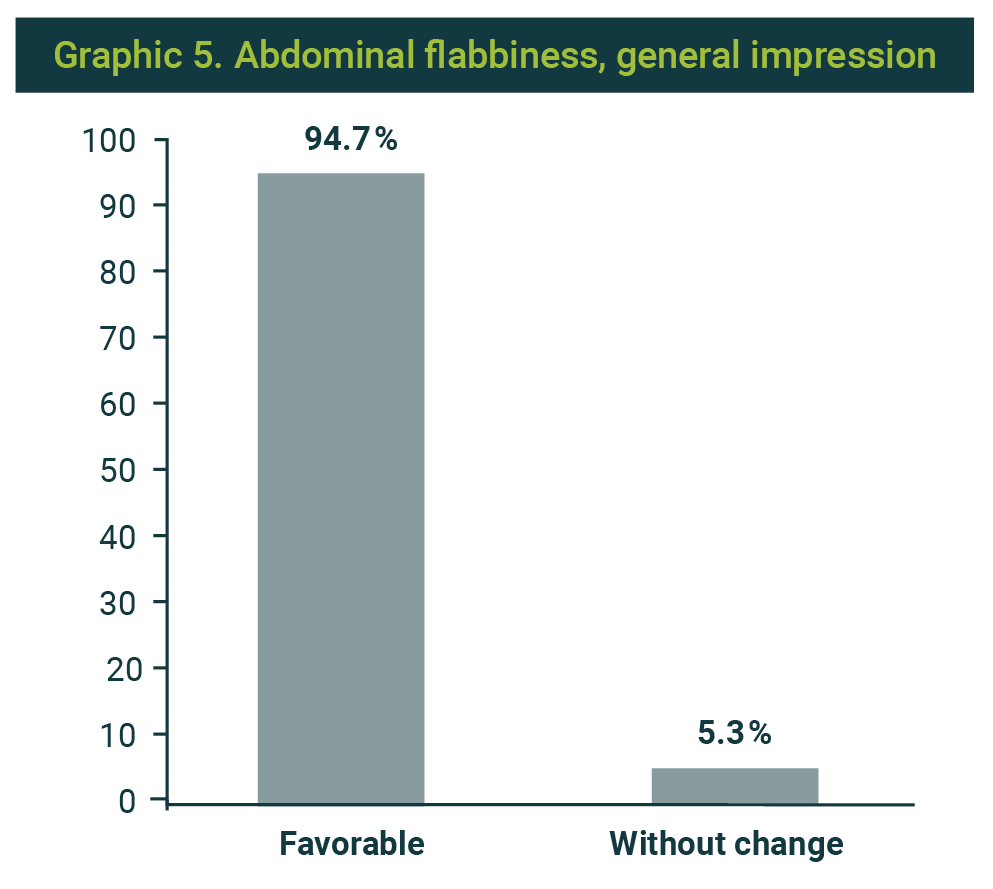
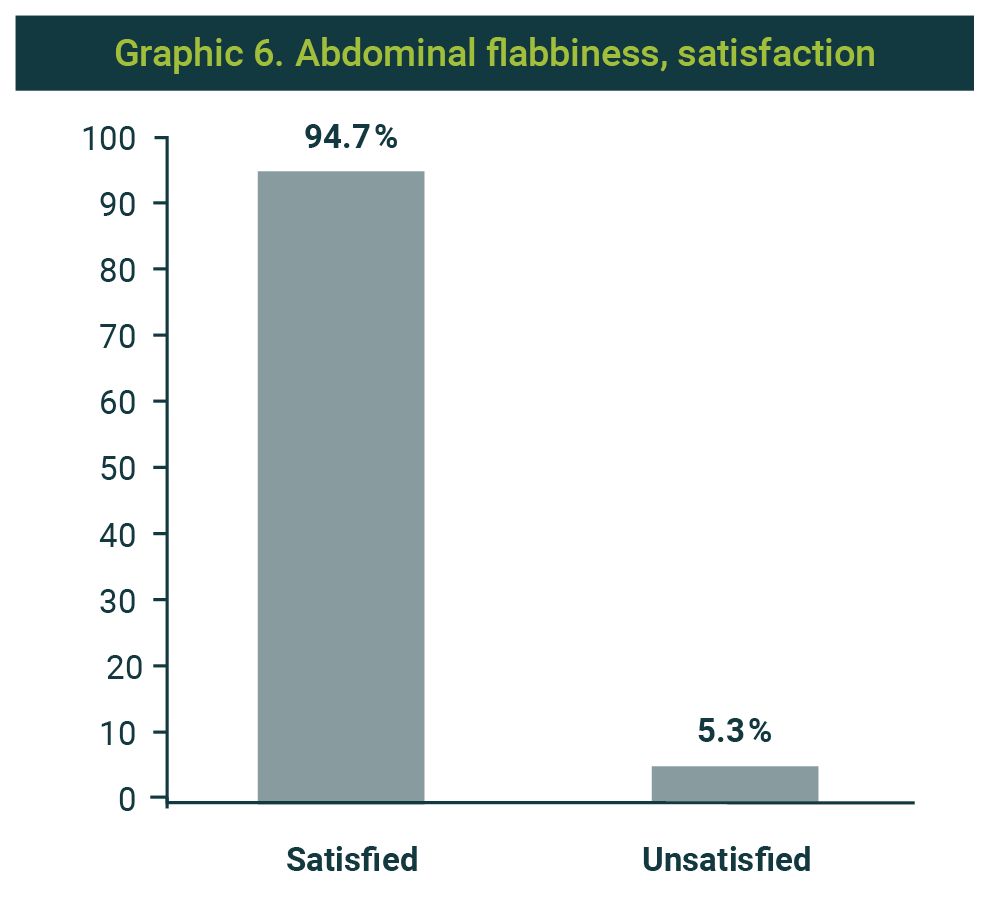
The result was favorable in 18 patients (94.7%), and only one reported without change (Graphic 5). Likewise, satisfaction was reported in 18 cases (94.7%) (Graphic 6).
Pictures 3 and 4 show examples of patients treated with enzymes for the management of abdominal flabiness.
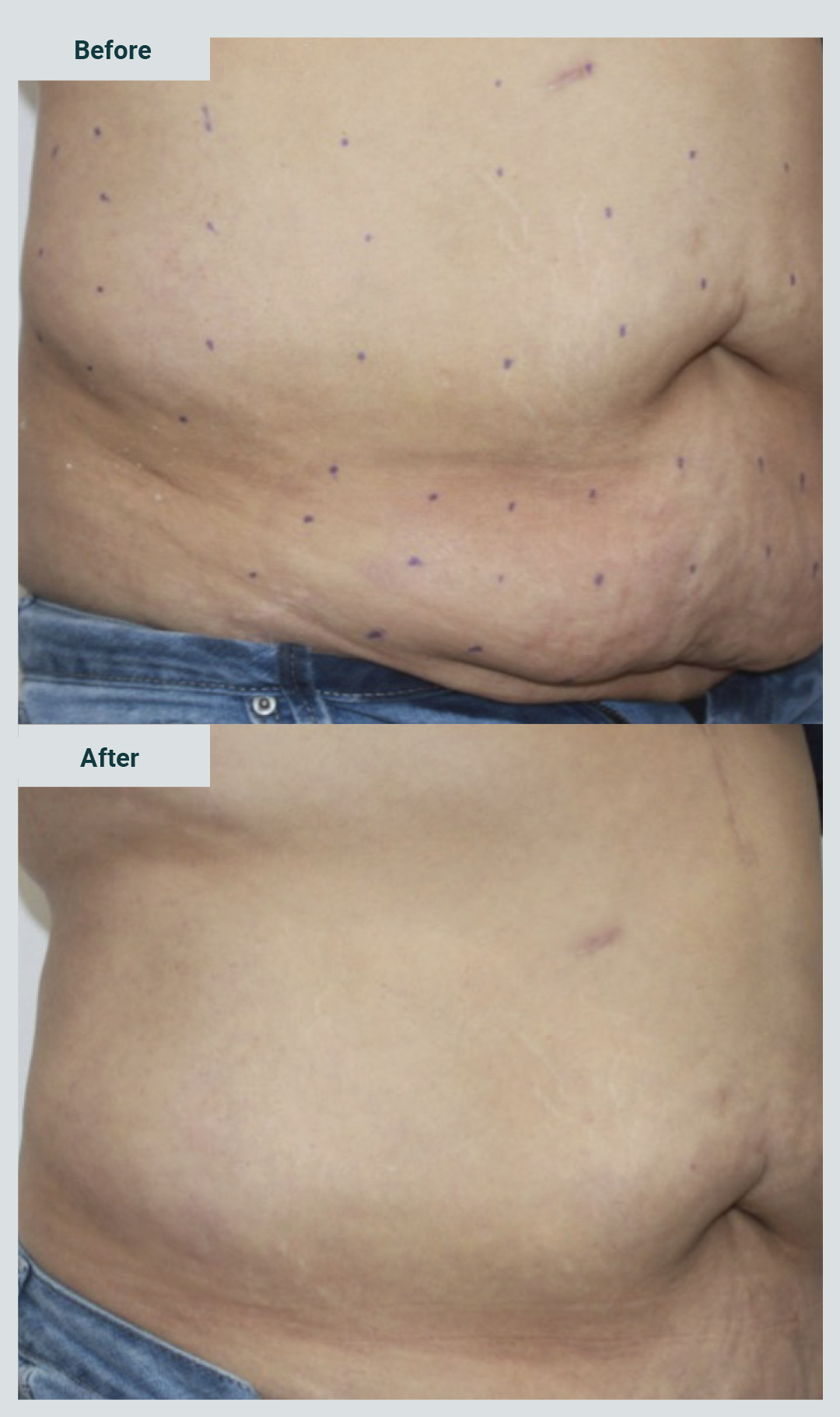
Limb flabbiness
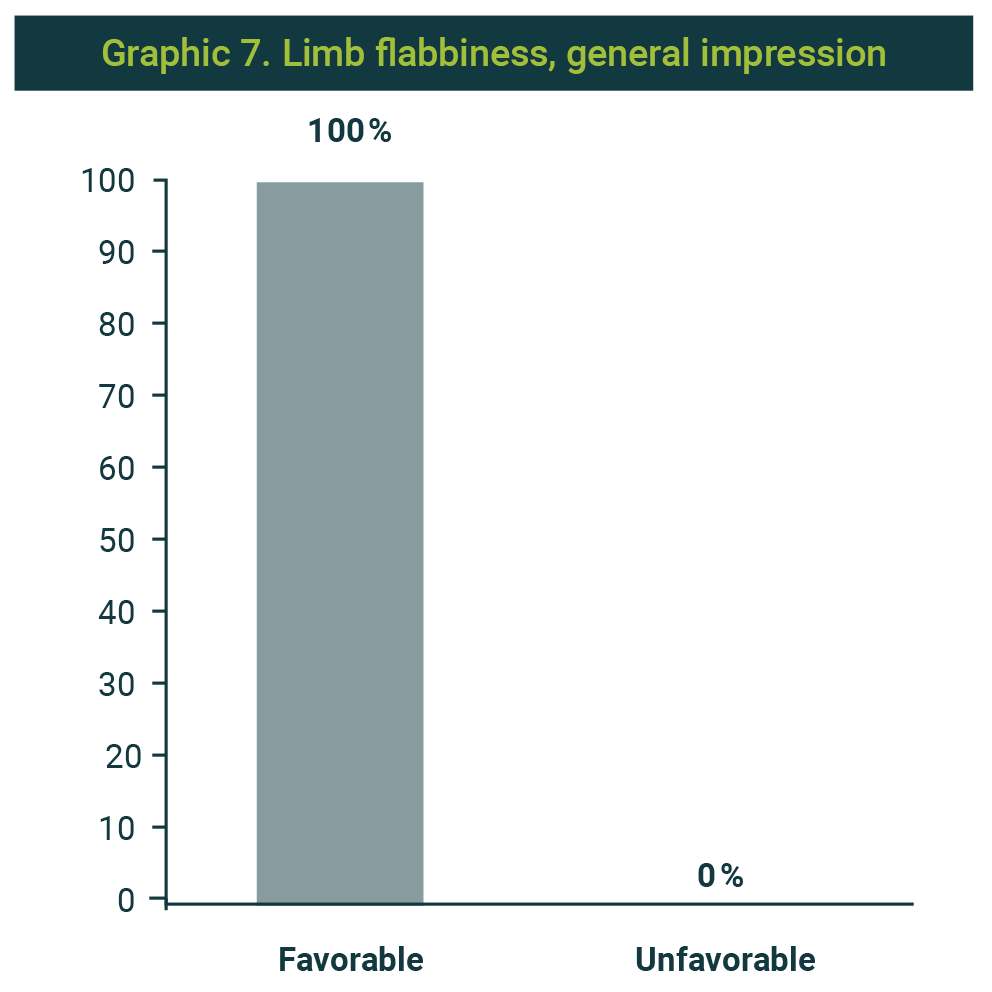
Seven cases were found with this indication in the series. They were aged 51.7 years on average, and all were women.
One case had a surgical history of liposuction and rhinoplasty. One patient reported being hypertense, and another one was allergic to sulfa. There was another case with a recent COVID-19 infection.
In all the cases, the combination of recombinant collagenase, hyaluronate lyase, and lipase was used. In six cases, a volume of 12 ml was used, and 15 ml was used only in one case. The required sessions were 4 in 6 cases and 5 in only one case. The interval between sessions was one week in all cases.
The change was assessed as favorable in all cases (Graphic 7), as it was satisfactory also (Graphic 8).

Combined treatment
Eight cases of combined treatment in the same session were included. They were jowl and double chin (7 cases) and nasolabial fat pad and jowl (1 case). The mean age was 46.3 years; only 1 patient was a man, and only 1 had a relevant surgical history (cheek lift). One patient reported having hypertension, another one had allergic rhinitis, 1 had recent COVID-19 infections, and 1 had hypothyroidism.
The combination of recombinant pbserum collagenase, hyaluronate lyase, and lipase was used in all the cases. The most frequently used volume was 9 ml (50%); in one case, 4 ml, 8.5ml in another, and 15 ml in another. Two cases had three sessions, and 6 cases required two. In 7 cases, the interval between sessions was one week. All 8 cases were assessed as favorable change and satisfactory.
Pbserum medium’s liase is obtained from Streptococcus pyogenes, and its function is to degrade proteoglycans. It enhances the tissue permeability and the penetration of collagenase and lipase. It also degrades the molecules responsible for liquid and toxins retention in the extracellular matrix.3
Collagenases are enzymes that can break collagen’s peptide links. They belong to the matrix metalloproteinases family; they participate in physiologic processes that support collagen’s integrity or rearrangement. Its primary function is the normal collagen replacement, but its activity increases to achieve the remodeling of the extracellular matrix during wound healing.
In therapeutics, collagenases treat burns and ulcers and remove scar tissue. They have a role mainly in the dermic repair process and help in the granulation and re-epithelization stage.3
Lipases are a group of enzymes that have the function of the hydrolysis of triglycerides to originate free fatty acids and glycerol. In humans, lipase activity is determined by hormone-dependent metabolism of fatty acids, diet habits, and physical activity. Some of its applications are as additives for detergents, cosmetics production, drugs, environmental treatments, and are used in the food industry.3 These enzymes are currently available as recombinant enzymes.
Based on their effects on tissues and our findings, it is suggested that recombinant enzymes (pbserum) in managing the indications studied in clinical practice in aesthetic dermatology and medicine are highly satisfactory for the patient’s body and face. Besides, their results are well evaluated by treating doctors.
In this series of 229 Latin American cases, a high degree of satisfaction is shown in the management of double chin, jowl, abdominal and limb flabbiness, as well as a good aesthetic result with the use of recombinant collagenase, hyaluronidase, and lipase combination. There were no reports of serious adverse effects or complications from the management with recombinant pbserum medium enzymes in this series of patients.
Enzymatic therapy is a new method used in aesthetic dermatology and medicine that uses the injection of enzymes such as lipase, collagenase, hyaluronidase, and lipase in the skin and subcutaneous tissue to reduce local fat and remodel collagen. This is the first series of patients reported regarding the use of this therapeutic tool in aesthetic dermatology and medicine that evaluates the satisfaction perception indexes for the treatment.
Since there are no backgrounds in the literature of publications about the effectiveness and safety of the management of localized fat with this pbserum enzymes, we consider this is a good first evidence that documents their use, which is extensive in medical practice.
The results presented here reflect daily practice in real life. Still, more prospective, quantitative, controlled, and comparative research is needed.
All patients signed an informed consent form and permitted to use data and images. Axios Pharma sponsored this study. Jorge López Berroa Works at Proteos Biotech, a manufacturer of pbserum medium. The rest of the authors do not express conflict of interest.
| 1. | Sahu S. Biotechnology for sustainable utilization of bioresources. 1st. ed. Delhi: Daya Publishing House; 2019. pp. 7-8. |
| 2. | Bhatia S, Goli D. Introduction to pharmaceutical biotechnology. Volume 1. Bristol UK: IOP Publishing; 2018. pp. 27-40. |
| 3. | Fierro-Arias L, Campos-Cornejo NG, Contreras-Ruiz J, Espinosa-Maceda S, López-Gehrke I, Márquez-Cárdenas R, et al. Productos enzimáticos (hialuronidasa, colagenasa y lipasa) y su uso en Dermatología. Dermatol Rev Mex. 2017;61(3):206-219. |
| 4. | Rivera PZM. Uso de enzimas como tratamiento dermatológico regenerador de las líneas de expresión. Revista de Salud Vive. 2020;3(8):77-84. |
| 5. | Monografía de producto PbSerum. [Consultado 26 de febrero, 2024]. Disponible en URL: https://pbserum.com/wp-content/uploads/2022/07/BAJA%20MONOGRAFIA%20LATAM.pdf |
| 6. | Zappelli C, Barbulova A, Apone F, Colucci G. Effective active ingredients obtained through biotechnology. Cosmetics. 2016;3(4):39. |
| 7. | Concept Clinic. Enzymne therapy–removal of double chin, sunken body contours and scars. Bratislava: Concept Clinic; 2022. [Retrieved on February 26, 2024]. Available from URL: https://cclinic.eu/medical/aesthetic-dermatology/enzyme-therapy-removal-of-double-chins-sunken-body-contours-and-scars/ |
All Rights Reserved® 2019
Latin American Journal of Clinical Sciences and Medical Technology,Año 1, No. 1, octubre, 2019 es una publicación contínua editada por Vesalio S.C.; http://www.lajclinsci.com/ • Editor responsable: Gilberto Castañeda Hernández. • Reserva de Derechos al Uso Exclusivo: 04-2019-062013242000-203; ISSN: 2683-2291; ambos otorgados por el Instituto Nacional del Derecho de Autor. • Responsable de la última actualización de este número, Web Master Hunahpú Velázquez Martínez,
Calle San Luis Potosí #182-1, Col. Roma, Alcaldía Cuauhtémoc, C.P. 06700, Ciudad de México; teléfono: 55 64 40 41 • Fecha de última modificación, 30 de marzo de 2020.
All Rights Reserved® 2019
Año 1, No. 1, octubre, 2019 es una publicación contínua editada por Vesalio S.C.; http://www.lajclinsci.com/ • Editor responsable: Gilberto Castañeda Hernández. • Reserva de Derechos al Uso Exclusivo: 04-2019-062013242000-203; ISSN: 2683-2291; ambos otorgados por el Instituto Nacional del Derecho de Autor. • Responsable de la última actualización de este número, Web Master Hunahpú Velázquez Martínez,
Calle San Luis Potosí #182-1, Col. Roma, Alcaldía Cuauhtémoc, C.P. 06700, Ciudad de México; teléfono: 55 64 40 41 • Fecha de última modificación, 30 de marzo de 2020.