| ** | Latin American Journal of Clinical Sciences and Medical Technology is an open access magazine. To read all published articles and materials you just need to register Registration is free of charge. Register now If you already have registered please Log In | ** |
aNeurology and Psychiatry Department, Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”, Mexico City, Mexico.
Corresponding Author: , . Telephone number: ; e-mail: erwinchiquete@hotmail.com
Lat Am J Clin Sci Med Technol. 2020 May;2:44-48.
Received: April 23rd, 2020.
Accepted: April 29th, 2020.
Published: May 1st, 2020.
Views: 1551
Downloads: 14
Introduction. Coronavirus disease 2019 (COVID-19) is the systemic entity caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that may cause death through severe atypical pneumonia and acute lung injury. Valproic acid (VPA) has shown anti-inflammatory activity and intrinsic antiviral effect. These properties warrant the study of VPA as a possible active treatment in people with severe COVID-19. Methods. Consecutive adult patients needing invasive mechanical ventilation (IMV) will be given intravenous (IV) VPA at a starting dose of 20 mg/kg/day and up to 60/kg/day (in 60 min IV infusions in 250 mL normal saline) as needed to reach plasma VPA concentrations of 50-100 µg/mL (measured every 72 h). These patients will be followed-up for 10 days for the primary outcome and for a further period of 30 days after treatment completion for the secondary outcome of recurrence. The primary study outcome is the reduction in the case fatality rate (CFR) of at least 50% after 10 days of treatment (as compared with natural history). Secondary outcomes are the reduction of length of stay (LOS) of at least 50%, as well as COVID-19 recurrence at 30-day follow-up. The most important safety outcomes are acute liver failure, acute pancreatitis, and thrombocytopenia. Conclusions. Although long-term adverse effects and even pro-inflammatory consequences have been reported with the chronic use of VPA, the study of VPA is justified from a scientific standpoint given the urgent need for a drug against COVID-19 to shorten the high mortality and LOS.
Introducción. La enfermedad por coronavirus 2019 (COVID-19) es la entidad sistémica causada por el coronavirus 2 del síndrome respiratorio agudo severo (SARS-CoV-2) que puede causar la muerte por neumonía atípica grave y lesión pulmonar aguda. El ácido valproico (AVP) ha demostrado actividad antinflamatoria y un leve efecto antiviral intrínseco. Estas propiedades justifican el estudio de AVP como un posible tratamiento activo en personas con COVID-19 grave. Métodos. Pacientes adultos consecutivos que necesiten ventilación mecánica invasiva (VMI) recibirán AVP intravenoso (i.v.) a una dosis inicial de 20 mg/kg/día y hasta 60/kg/día (en infusiones i.v. de 60 minutos en solución salina normal de 250 ml) según sea necesario para alcanzar concentraciones plasmáticas de AVP de 50-100 µg/mL (medidas cada 72 h). Estos pacientes serán seguidos durante 10 días para el desenlace primario y por un período adicional de 30 días después de la finalización del tratamiento para el desenlace secundario de recurrencia. El desenlace primario del estudio es la reducción de la letalidad de al menos 50% después de 10 días de tratamiento (en comparación con la historia natural). Los desenlaces secundarios son la reducción de la duración de la estancia hospitalaria de al menos 50%, y la recurrencia de COVID-19 a los 30 días de seguimiento. Los desenlaces de seguridad más importantes son insuficiencia hepática aguda, pancreatitis aguda y trombocitopenia. Conclusiones. Aunque se han informado efectos adversos a largo plazo e incluso consecuencias proinflamatorias con el uso crónico de AVP, dada la necesidad urgente de un medicamento contra COVID-19 para acortar la alta mortalidad y la estancia hospitalaria, el estudio de AVP está justificado desde el punto de vista científico.
Coronavirus disease 2019 (COVID-19) is the systemic disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that may cause death through severe atypical pneumonia and acute lung injury. COVID-19 has no effective vaccine or cure, and several drug therapies have shown limited efficacy. Thus, there is a need for useful molecules that cut the rate of deaths and hospital length of stay (LOS) causing a high sanitary burden. Given the urgent need for high-efficacy drugs, repurposed molecules may be the most efficient approach given the scarce time to limit the sanitary and economic impact of the present pandemics.
Valproic acid (VPA, the conjugate base is valproate) is available as sodium, semi sodium, and magnesium. VPA is a drug primarily used to treat epilepsy, bipolar disorder and migraine.1 Chemically, VPA is an organic weak acid that was first synthesized in 1882 as an analog of valeric acid, found naturally in the plant Valeriana officinalis.1,2 VPA was first tested initially as a solvent of organic compounds intended and in 1962 serendipitously found to be active in animals and humans as an anticonvulsant.3 It is a carboxylic acid clear liquid at room temperature. It was first approved to be used as a medical compound in humans in France in 1967, specifically as an anti-epileptic medication.1
VPA attenuates the expression of pro-inflammatory cytokines in preclinical models4-9 and humans10,11, especially during the acute treatment phase, although conflicting results have been reported.12 This molecule has also shown intrinsic antiviral activity probably due to its activity as histone deacetylase inhibitor.9,13-16 Moreover, VPA has shown anti-inflammatory activity in myocardial17 as well as neural tissue in models of brain and spinal cord injuries18,19, even though some conflicting findings.20
Although long-term adverse effects and even pro-inflammatory consequences have been reported with the chronic use of VPA,12 we believe that, given the urgent need to have a useful drug to shorten the high mortality and LOS associated with COVID-19,21,22 the study of the safety and efficacy of VPA in the treatment of COVID-19 is warranted from a scientific standpoint.
Study Type
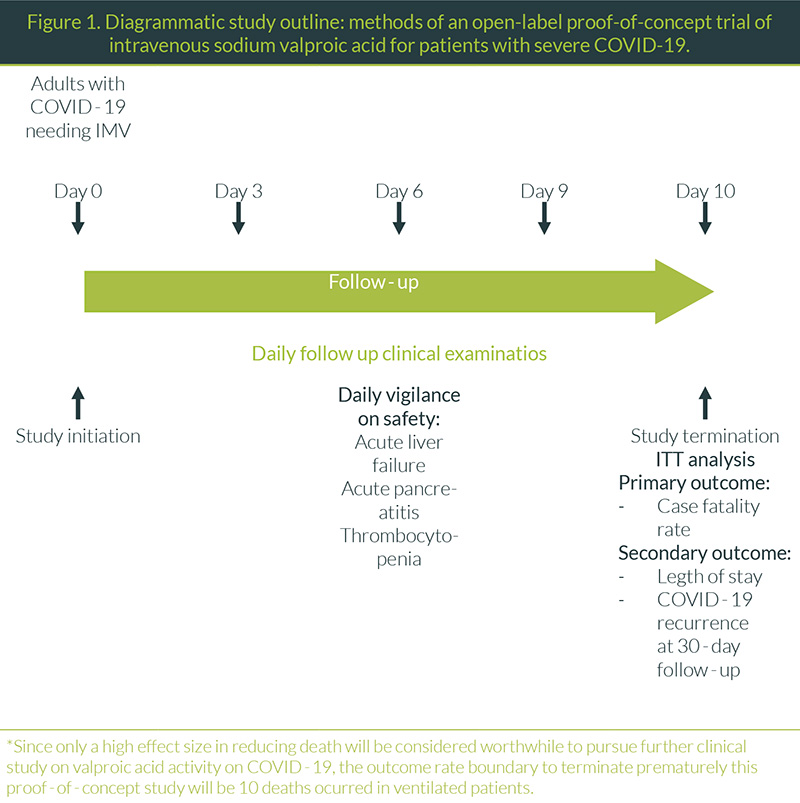
Open-label proof-of-concept interventional study (Figure 1).
Hypothesis
Intravenous (IV) valproic acid will be safe and effective in the treatment of patients with severe COVID-19.
Intervention
Sodium valproic acid will be given intravenously at a starting dose of 20 mg/kg/day and up to 60/kg/day as needed to reach plasma valproic acid concentration of 50-100 µg/mL (measured every 72 h). Three equal doses per day given intravenously in infusions lasting 60 min in 250 mL normal saline.
Study Oversight and Ethical Considerations
Ten days of IV treatment for the primary outcome, plus 30 days for the secondary outcome of COVID-19 recurrence, given a total of 40 days of follow-up.
Population
Patients admitted with severe COVID-19 to the ICU of Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” (INCMNSZ).
Main Study Outcomes
Primary outcomes
- Reduction in a case fatality rate (CFR) of at least 50%, from a known proportion of 40% to 20% after 10 days of treatment.
Secondary exploratory outcomes
- Reduction of length of stay (LOS) of at least 50%, from a known median LOS of 10 days to 5 days after at least 5 days of IV valproic acid exposition.
- No COVID-19 recurrence during a 30-day observational period, after completion of the 10-day IV therapy.
Selection Criteria
Inclusion criteria
- Patients aged >18 years
- Both sexes
- Positive to SARS-CoV-2 RNA in nasopharyngeal samples by RT-PCR
- Severe acute respiratory syndrome treated with invasive mechanical ventilation (IMV) at study recruitment
- Any time elapsed since symptoms onset to study recruitment
- Any length of hospital stay
- Any length of IMV
- Signed informed consent from the patient or legal proxy
- No known VPA allergy
- Patients with or without previous or current exposure to anti-epileptics
- Patients with or without previous or current exposure to chloroquine, hydroxychloroquine or azithromycin
- Patients not participating in an interventional trial
- Patients not under current valproate treatment
- Patients not under current treatment with remdesivir
- Patients not under current treatment with tocilizumab
Exclusion criteria
- Decline of informed consent
- Signs of VPA allergy
- Any acute severe side effect (either thrombocytopenia, acute liver disease, moderate-to-severe hypertransaminasemia, or acute pancreatic injury)
- Incomplete information for primary outcome *
*Intention-to-treat analysis (ITT) will be performed, considering study dropouts as failures.
Recruitment Strategy
Consecutive patients needing IMV and meeting selection criteria will be given IV valproic acid for 10 days maintaining a plasma concentration of 50-100 µg/mL (measured every 72 h). These patients will be followed-up for 10 days for the primary outcome and a further period of 30 days after treatment completion.
Safety Outcomes
The most important acute side effects of valproic acid that should be meticulously daily examined are:
- Acute liver failure, defined as the severe acute liver injury with encephalopathy and impaired synthetic function (international normalized ratio ≥1.5) in a subject free of preexistent chronic liver disease.
- Acute pancreatitis, defined as the American Gastroenterological Association (AGA) guidelines as the presence of two out of three of the following characteristics: a) abdominal pain consistent with the disease, b) serum amylase and/or lipase activity >3 times the upper limit of normal, and c) characteristic findings on abdominal imaging. 23
- Thrombocytopenia, defined as the platelet blood count <50,000 per mL.
- Hypertransaminasemia, defined as the elevation of alanine transaminase (ALT) and/or aspartate transaminase (AST) activity in serum, >3 times the upper normal limit of the laboratory reference.
As stated in the selection criteria, patients with the current use of anti-epileptics are allowed to be recruited, criteria that may include patients with epilepsy. In case of the need for other anti-epileptics to treat new or recurrent seizures, the inclusion of such medications will be a prerogative of the treating physician team. The investigational team of this trial will reinforce the maintenance of the patient in the study, by helping in the selection of anti-epileptics with no known adverse interactions with VPA, but in any particular case of perceived harm that may arise with the interaction of VPA with other anti-epileptics, the patient will be removed from the study.
Study Duration
Ten days of IV treatment for the primary outcome, plus 30 days for the secondary outcome of COVID-19 recurrence, given a total of 40 days of follow-up.
Major Statistical Hypothesis
There will be at least a 50% reduction of death of patients with severe COVID-19 needing IMV.
Sample Size
This is a proof-of-concept study; thus, sample size calculation was performed by using the formula for the difference of proportions with a known (previous) proportion, and assuming a high magnitude of the efficacy of the study drug, since, given the contingency of this pandemic, minor effect sizes reductions are not desirable.
According to the most extensive information available today on 1,591 Italian patients with severe COVID-19 admitted to ICU24, it is expected a 40% CFR final disposition of patients, according to Bayesian analysis (the original information reports 26% CFR, but 58% of patients still in ICU as of the analysis deadline of March 25th, 2020). Hence, the sample size was calculated to detect a minimum of (but not restricted to) 50% reduction in the CFR from 40% to 20%, with 80% study power and 5% type I error.
Therefore, a sample size of at least (but not restricted to) 43 patients will be needed to demonstrate an absolute (not RRR) reduction of 50% in CFR. No excess sample size was considered to cover study losses since ITT analysis requires that drop-outs are considered as failures to achieve study outcomes.
Statistical Plan
ITT analyses will be performed considering all patients removed from the study for any reason as treatment failures. Per protocol (PP) analyses will also be performed considering in the calculations only patients with complete information and complete treatment schemes, but the analyses on the PP population will only be considered as hypothesis-generating to further pursue the study objectives with another robust design. Daily patients inspections will be performed to demonstrate that valproic acid is not harmful, to review plasma levels (measured every 72 h), and clinical response. With this information, daily statistical analyses will be performed for outcome differences by using chi-squared tests for categorical variables and hazard ratios to obtain effect sizes of study outcomes (primary and secondary outcomes), as well as safety outcomes. After completion of the study (either as per protocol or prematurely terminated), actuarial analyses with the Kaplan-Meier method will be performed for each study outcome to illustrate effect differences. All analyses performed will be two-tailed and wondered as significant when p<0.05.
Interim Analyses and Termination Rules
This study will be terminated as per protocol when at least 43 patients complete 10 days of IV valproic acid therapy.
Premature termination will be executed when the following events occur:
- Since only a high effect size (>50%) in reducing death will be considered worthwhile to pursue further clinical study on valproic acid activity on COVID-19, the outcome rate boundary to terminate prematurely this proof-of-concept study will be 10 deaths occurred in ventilated patients.
- A rate of acute side effects (either thrombocytopenia, acute liver disease or acute pancreatic injury) exceeding 4 cases (10% event rate) will be considered unacceptable and will lead to premature termination, given that this rate exceeds the expected, given the current information on critically-ill patients exposed to valproic acid to treat epilepsy and status epilepticus.
A useful drug must be effective to treat COVID -19 and its consequences. Given that it is expected the duration of this pandemic is long25, it can be supposed that we will have several ways to treat this disease to improve clinical outcomes of crucial importance to eventually stop the direct sanitary implications and the very high economic burden that the COVID-19 pandemic has caused. Although long-term adverse effects and even pro-inflammatory consequences have been reported with the chronic use of VPA, given the urgent need for a drug active against COVID-19 to shorten the high mortality and LOS, the study of VPA is justified from a scientific standpoint.
The authors are grateful to the authorities, colleagues, and patients of Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” for their support to perform this trial, as well as the grants of possible donors that are currently evaluating this investigation idea.
The authors have no relevant conflicts of interest to declare.
This registry received no funding.
| 1. | Löscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16(10):669-94. |
| 2. | Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16. |
| 3. | Terbach N, Williams RS. Structure-function studies for the panacea, valproic acid. Biochem Soc Trans 2009; 37: 1126–32. |
| 4. | Amirzargar MA, Yaghubi F, Hosseinipanah M, Jafari M, Pourjafar M, Rezaeepoor M, et al. Anti-inflammatory Effects of Valproic Acid in a Rat Model of Renal Ischemia/Reperfusion Injury: Alteration in Cytokine Profile. Inflammation. 2017 Aug;40(4):1310-1318. doi:10.1007/s10753-017-0574-9. |
| 5. | Hoşgörler F, Keleş D, Tanrıverdi-Akhisaroğlu S, İnanç Ş, Akhisaroğlu M, Cankurt Ü, et al. Anti-inflammatory and Anti-apoptotic Effect of Valproic Acid and Doxycycline Independent from MMP Inhibition in Early Radiation Damage. Balkan Med J. 2016 Sep;33(5):488-495. |
| 6. | Delgado FG, Cárdenas P, Castellanos JE. Valproic Acid Downregulates Cytokine Expression in Human Macrophages Infected with Dengue Virus. Diseases. 2018 Jul 6;6(3). pii: E59. doi: 10.3390/diseases6030059. |
| 7. | Seet LF, Toh LZ, Finger SN, Chu SWL, Wong TT. Valproic acid exerts specific cellular and molecular anti-inflammatory effects in post-operative conjunctiva. J Mol Med (Berl). 2019 Jan;97(1):63-75. doi: 10.1007/s00109-018-1722-x. |
| 8. | Kasotakis G, Galvan MD, Osathanugrah P, Dharia N, Bufe L, Breed Z, et al. Timing of valproic acid in acute lung injury: prevention is the best therapy? J Surg Res. 2017 Dec;220:206-212. doi: 10.1016/j.jss.2017.06.088. |
| 9. | Soria-Castro R, Schcolnik-Cabrera A, Rodríguez-López G, Campillo-Navarro M, Puebla-Osorio N, Estrada-Parra S, et al. Exploring the Drug Repurposing Versatility of Valproic Acid as a Multifunctional Regulator of Innate and Adaptive Immune Cells. J Immunol Res. 2019 Mar 14;2019:9678098. doi: 10.1155/2019/9678098. |
| 10. | Steinborn B, Zarowski M, Winczewska-Wiktor A, Wójcicka M, Młodzikowska-Albrecht J, et al. Concentration of Il-1β, Il-2, Il-6, TNFα in the blood serum in children with generalized epilepsy treated by valproate. Pharmacol Rep. 2014 Dec;66(6):972-5. doi: 10.1016/j.pharep.2014.06.005. |
| 11. | Sonmez FM, Serin HM, Alver A, Aliyazicioglu R, Cansu A, Can G, et al. Blood levels of cytokines in children with idiopathic partial and generalized epilepsy. Seizure. 2013 Sep;22(7):517-21. doi: 10.1016/j.seizure.2013.03.014. |
| 12. | Shiah IS, Yatham LN, Yeh CB, Ravindran AV. Effect of valproate on plasma levels of interleukin-6 in healthy male humans. Int Clin Psychopharmacol. 2005 Nov;20(6):295-8. |
| 13. | Crespillo AJ, Praena B, Bello-Morales R, Lerma L, Vázquez-Calvo A, Martín-Acebes MA, et al. Inhibition of herpes virus infection in oligodendrocyte cultured cells by valproic acid. Virus Res. 2016 Mar 2;214:71-9. doi: 10.1016/j.virusres.2016.01.009. |
| 14. | Vázquez-Calvo A, Saiz JC, Sobrino F, Martín-Acebes MA. Inhibition of enveloped virus infection of cultured cells by valproic acid. J Virol. 2011 Feb;85(3):1267-74. doi: 10.1128/JVI.01717-10. |
| 15. | Vázquez-Calvo Á, Martín-Acebes MA, Sáiz JC, Ngo N, Sobrino F, de la Torre JC. Inhibition of multiplication of the prototypic arenavirus LCMV by valproic acid. Antiviral Res. 2013 Aug;99(2):172-9. doi: 10.1016/j.antiviral.2013.05.012. |
| 16. | Praena B, Bello-Morales R, de Castro F, López-Guerrero JA. Amidic derivatives of valproic acid, valpromide and valnoctamide, inhibit HSV-1 infection in oligodendrocytes. Antiviral Res. 2019 Aug;168:91-99. doi:10.1016/j.antiviral.2019.05.006. |
| 17. | Jin H, Guo X. Valproic acid ameliorates coxsackievirus-B3-induced viral myocarditis by modulating Th17/Treg imbalance. Virol J. 2016 Oct 10;13(1):168. |
| 18. | Chen S, Ye J, Chen X, Shi J, Wu W, Lin W, et al. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-ΚB pathway dependent of HDAC3. J Neuroinflammation. 2018 May 18;15(1). |
| 19. | Chen X, Wang H, Zhou M, Li X, Fang Z, Gao H, et al. Valproic Acid Attenuates Traumatic Brain Injury-Induced Inflammation in Vivo: Involvement of Autophagy and the Nrf2/ARE Signaling Pathway. Front Mol Neurosci. 2018 Apr 17;11:117. doi: 10.3389/fnmol.2018.00117. |
| 20. | Dambach H, Hinkerohe D, Prochnow N, Stienen MN, Moinfar Z, Haase CG, Hufnagel A, Faustmann PM. Glia and epilepsy: experimental investigation of antiepileptic drugs in an astroglia/microglia co-culture model of inflammation. Epilepsia. 2014 Jan;55(1):184-92. doi: 10.1111/epi.12473. |
| 21. | Noël F Lima J. Pharmacological aspects and clues for the rational use of Chloroquine/Hydroxychloroquine facing the therapeutic challenges of COVID-19 pandemic. Lat Am J Clin Sci Med Technol [Internet]. 2020 [cited 2020 Apr 28];2:28–34. Available from: http://lajclinsci.com/vD?tD=3&rG=5&tpA=ver |
| 22. | Zuluaga AF, Rodríguez CA M-GM. Which is the pharmacodinamic target to calculate a rational dose against SARS-CoV-2? Lat Am J Clin Sci Med Technol [Internet]. 2020;2:35–7. Available from: http://www.lajclinsci.com/vD?tD=4&rG=2&tpA=ver |
| 23. | Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018 Mar;154(4):1096-1101. |
| 24. | Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 Apr 6. doi: 10.1001/jama.2020.5394. [Epub ahead of print] |
| 25. | Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020 Apr 14. pii: eabb5793. doi: 10.1126/science.abb5793. |
All Rights Reserved® 2019
Latin American Journal of Clinical Sciences and Medical Technology,Publicación contínua • Editor responsable: Gilberto Castañeda Hernández. • Reserva de Derechos al Uso Exclusivo: 04-2019-062013242000-203; ISSN: 2683-2291; ambos otorgados por el Instituto Nacional del Derecho de Autor. • Responsable de la última actualización de este número, Web Master Hunahpú Velázquez Martínez,
Calle Profesor Miguel Serrano #8, Col. Del Valle, Alcaldía Benito Juárez, CP 03100, Ciudad de México, México. Número telefónico: 55 5405 1396 • Fecha de última modificación, 28 de agosto de 2024.
All Rights Reserved® 2019
Publicación contínua • Editor responsable: Gilberto Castañeda Hernández. • Reserva de Derechos al Uso Exclusivo: 04-2019-062013242000-203; ISSN: 2683-2291; ambos otorgados por el Instituto Nacional del Derecho de Autor. • Responsable de la última actualización de este número, Web Master Hunahpú Velázquez Martínez,
Calle Profesor Miguel Serrano #8, Col. Del Valle, Alcaldía Benito Juárez, CP 03100, Ciudad de México, México. Número telefónico: 55 5405 1396 • Fecha de última modificación, 28 de agosto de 2024.